Continue to the subsequent area to dive further into some great benefits of the document management technique during the pharmaceutical marketplace.
A full-fledged DMS should provide personnel of pharma corporations with effortlessly produced stories. The kinds from the reviews may possibly fluctuate, from the stock status during the warehouse to profits, according to the pharma organization’s action.
Don’t involve individual or economic details like your Countrywide Insurance amount or bank card information.
Making certain that each one output deviations are described and evaluated Which significant deviations are investigated and also the conclusions are recorded
For each review or trial explained inside the write-up marketing prerequisite/commitments data files a bookmark needs to be incorporated.
Making sure that there's stability data to guidance retest or expiry dates and storage situations on APIs and/or intermediates, the place correct
Subsequently, continuously rising digital trends throughout the sector grew to become the driving force for varied pharma organizations that abide by them to improve their capabilities.
Now Allow’s look at the crucial characteristics for a robust electronic document administration procedure for your pharmaceutical field.
” The EMA draft guideline states “no less than 3 consecutive batches,” with justification for being offered (there are several exceptions into the current assertion).
As You may even see, the development of a pharmaceutical doc management system isn't a bit of cake. On the other hand, the the right way selected method of its progress along with the profound comprehension of the sector’s needs and pitfalls may assist make a really-functional Alternative that should here allow paperless doc management, files protection, and compliance.
The software will go well with. PrimeDMS can be used as part of the program package deal leveraged in the pharmacy. It can help pharmacists of varied dimensions digitize and deal with documents, patient ID playing cards, prescriptions, insurance policy statements, and other types of documents connected with pharmacy functions.
Ans: The clean up hold time is defined as the time in between the completion of cleansing as well as initiation of the subsequent production Procedure.
Frequent excellent-opinions of APIs needs to be carried out with the target of verifying the regularity of the method. These kinds of opinions really should Ordinarily be executed and documented yearly and may include at the least:
Ans: Expiry day: The date placed on the container/labels of the API designated the time all through which the API is anticipated to remain in proven shelf lifetime specifications if stored underneath pre-outlined conditions and following that, it should not be advised website for use.
 Jaleel White Then & Now!
Jaleel White Then & Now! Bradley Pierce Then & Now!
Bradley Pierce Then & Now! Danielle Fishel Then & Now!
Danielle Fishel Then & Now!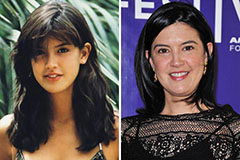 Phoebe Cates Then & Now!
Phoebe Cates Then & Now!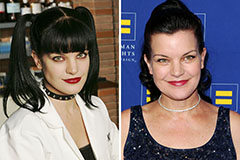 Pauley Perrette Then & Now!
Pauley Perrette Then & Now!